Type of resources
Available actions
Topics
Keywords
Contact for the resource
Provided by
Years
Formats
Representation types
Update frequencies
status
Service types
Scale
Resolution
-
During National Science Week on Sunday 26th August 2007, Geoscience Australia opened its doors to the community to showcase a diverse range of work activities. Members of the public had the opportunity to discover how earthquakes are detected, pan for gold, tour the building, view Australia in 3D, become a seafloor detective and talk to the people who work for Australia's national geoscience research organisation. The photographs of that open day have been converted into thumbmail images and are available on the GA web site.
-
This dataset is the definitive set of locality boundaries for the state of Victoria as defined by Local Government and registered by the Registrar of Geographic Names. The boundaries are aligned to Vicmap Property. This dataset is part of the Vicmap Admin dataset series.
-
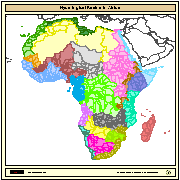
Major hydrological basins and their sub-basins. This dataset divides the African continent according to its hydrological characteristics. The dataset consists of the following information:- numerical code and name of the major basin (MAJ_BAS and MAJ_NAME); - area of the major basin in square km (MAJ_AREA); - numerical code and name of the sub-basin (SUB_BAS and SUB_NAME); - area of the sub-basin in square km (SUB_AREA); - numerical code of the sub-basin towards which the sub-basin flows (TO_SUBBAS) (the codes -888 and -999 have been assigned respectively to internal sub-basins and to sub-basins draining into the sea)
-
This data set was collected by sensors deployed as part of the Port Curtis Integrated Monitoring Program PCIMP - ZONE 06a - Calliope Estuary (lower).
-
This data consists of 25752 records derived from 63 tows covering 36.114 km at 1.40m resolution, over a depth range of .9 to 18.1 meters. Classification of the benthos and substrate was based on 17 benthic classes and 6 substrate classes. Summary of the primary benthic categories recorded Gorgonians 0.98% Hard Coral 11.57% Macroalgae 7.78% NoBenthos 39.82% Seagrass 16.32% Other 23.50% Summary of substrates recorded Rubble 0.63% NoSub 0.88% Rock 13.64% Sand 25.38% Silt 27.55% Sludge 5.91% Mud 23.23% Stone 1.30% SandCoarse 1.44% A custom interface has been developed by AIMS staff for reading Towed Video tapes.
-
This data consists of 14936 records derived from 36 tows covering 31.51 km at 2.11m resolution, over a depth range of 4.0 to 499.0 meters. Classification of the benthos and substrate was based on 17 benthic classes and 6 substrate classes. Summary of the primary benthic categories recorded Filter feeders 0.35% Gorgonians 48.90% Hard Coral 22.25% Macroalgae 6.70% NoBenthos 21.03% Soft Coral 0.74% Summary of substrates recorded Rubble 16.72% NoSub 0.04% Rock 15.12% Sand 27.04% Limestone 21.57% SandWaves 2.02% Stone 8.63% SandCoarse 8.82% A custom interface has been developed by AIMS staff for reading Towed Video tapes.
-
Towed Video - Benthic survey of West Warrego, Barcoo Bank, Karamea Bank (14-Feb-2007 - 16-Feb-2007 )
This data consists of 5418 records derived from 6 tows covering 10.421 km at 1.92m resolution, over a depth range of .7 to 50.1 meters. Classification of the benthos and substrate was based on 17 benthic classes and 6 substrate classes. Summary of the primary benthic categories recorded Gorgonians 26.55% Hard Coral 19.17% Macroalgae 24.19% NoBenthos 9.59% Soft Coral 20.46% Summary of substrates recorded Rubble 17.33% NoSub 0.03% Rock 3.24% Sand 7.05% Limestone 48.54% Silt 5.48% SandWaves 1.03% Stone 9.80% SandCoarse 7.47% A custom interface has been developed by AIMS staff for reading Towed Video tapes.
-
'Australian National Moorings Network' (ANMN) is a facility of the Australian 'Integrated Marine Observing System' (IMOS) project. This data set was collected by the ANMN sub-facility 'National Reference Systems' (NRS).
-
This data set was collected by sensors deployed as part of the Port Curtis Integrated Monitoring Program PCIMP - ZONE 12 - Colosseum Inlet.
-
This data set was collected by sensors deployed as part of the Port Curtis Integrated Monitoring Program PCIMP - ZONE 06b - Calliope Estuary (upper).
 AIMS Geonetwork
AIMS Geonetwork